 Indexed in Index Medicus and Medline
Indexed in Index Medicus and Medline
HOW I DO IT
The Rezūm system – a minimally invasive water vapor thermal therapy for obstructive benign prostatic hyperplasia
Christopher H. Cantrill, MD,1 Kevin C. Zorn, MD,2 Dean S. Elterman, MD,3 Ricardo R. Gonzalez, MD4
1Urology San Antonio, San Antonio, Texas, USA
2Department of Urology, University of Montreal Hospital Center, Montreal, Quebec, Canada
3Division of Urology, University Health Network, University of Toronto, Toronto, Ontario, Canada
4Houston Methodist Hospital, Houston, Texas, USA
CANTRILL CH, ZORN KC, ELTERMAN DS, GONZALEZ RR. The Rezum system – a minimally invasive water vapor thermal therapy for obstructive benign prostatic hyperplasia. Can J Urol 2019;26(3):9787-9793.
Benign prostatic hyperplasia (BPH) and accompanying lower urinary tract symptoms (LUTS) sits in the top ten prominent and costly disease conditions in men over 50 years of age. In the United States it is the most common diagnosis made by urologists for men 45 to 74 years of age. Twenty percent of the population will reach 65 years of age or older by 2030, and those over 85 years will represent the fastest growing segment of our population. The prevalence of symptomatic BPH increases proportionally with the aging population. It is estimated that BPH now affects 6% of the male population worldwide. Moreover, in Canada, the estimated BPH prevalence is more than
1 million men aged 50 years and older. Among the various surgical treatments, Rezum water vapor thermal therapy has been developed as a unique, rapid and reproducible minimally invasive surgical treatment exhibiting safe and early effective relief of LUTS/BPH. The targeted prostate tissue ablation is amenable to all zones of the prostate including intravesical median lobes. We present our experiences with this technique, which can be quickly performed under local anesthesia in an office setting.
Key Words: water vapor thermal therapy, Rezūm system, benign prostatic hyperplasia, prostate, LUTS, minimally invasive surgical treatment
Accepted for publication January 2019
A video clip is available online at www.canjurol.com
Address correspondence to Dr. Ricardo R. Gonzalez, Houston Methodist Hospital, 6560 Fannin Street, Suite 2100, Houston, TX 77030 USA
Introduction
The Rezūm system utilizing convective radiofrequency (RF) water vapor thermal therapy is a relatively new treatment for obstructive benign prostatic hypertrophy. The device received Conformité Européene (European Conformity, CE) marking in 2013, FDA approval in 2015 and Health Canada clearance in 2018. The Rezūm randomized controlled trial (RCT) demonstrated that a single transurethral procedure results in significant relief of lower urinary tract symptoms (LUTS), both storage and voiding functions, quality of life measures and urinary flow rates.1 Improvements of at least 50% in International Prostate Symptom Scores (IPSS) and maximum flow rate (Qmax) were observed as early as 2 weeks after the procedure. Sexual function including antegrade ejaculation was preserved.2 Durability reports demonstrate the reproducibility and stability of these results throughout 4 years. Similarly, erectile function remained improved in the subset of sexually active subjects.3-6 Men with a spectrum of LUTS of moderate severity (IPSS 8-18) or severe symptoms (IPSS 19-35) appear to achieve significant improvements. The Rezūm procedure is also applicable for ablation of the median lobe and enlarged central zone evidenced by an elevated bladder neck. This article shares our experience from clinical studies as well as community urology practice to detail intra-operative techniques that may guide clinicians new to this efficient and versatile minimally invasive thermal therapy.
Rationale for the Rezūm system technology
The Rezūm system (Boston Scientific Company Inc., Marlborough, MA, USA) water vapor thermal therapy has been well described previously.1,7,8 The principles of the technology and effective prostate tissue ablation were validated in early histological and magnetic resonance imaging studies.9,10 The Rezūm system is unique in the use of more efficient convective heat energy compared to historical tissue conductive heat transfer ablation procedures (transurethral needle ablation [TUNA] or transurethral microwave therapy [TUMT]). The latter induces molecular agitation within a tissue mass after direct contact between two surfaces at different temperatures; this causes a thermal gradient. Consequently, higher temperatures and longer periods of heating are required to achieve a therapeutic temperature in the target tissue via conduction versus convection. Moreover, with convective heating, there is instead a phase change of water vapor (steam at ~ 103° C) to liquid such that energy moves through the tissue interstices confined only by natural collagen barriers (adenoma pseudocapsule), limited to the McNeal’s transitional prostatic zone.11 This has been metaphorically likened to “the way wind blows against a sail without penetrating.”9 The target tissue temperature immediately reaches ~ 70° C resulting in irreversible and instantaneous cell death, creating a roughly spherical ablative lesion. No thermal effects occur outside of the prostate or in the peripheral zone when the transition zone is targeted. In addition, because vapor is wet thermal energy, there is no charring, desiccation or carbonization of the treated tissue. The Rezūm system and delivery device are shown in Figures 1 and 2.
Methods and techniques
Patient evaluation and confirmation for procedure candidacy
Compared with a formal clinical trial, clinicians possess flexibility in patient selection based upon variable prostate sizes, symptom severity, flow rate, and no morphological limitations, including patients with an obstructing median lobe and/or enlarged central zone.8 In general, any patient who is a candidate for a minimally invasive surgical therapy (MIST) is a candidate for Rezūm treatment. Compared with earlier MIST procedures such as prostate urethral lift (PUL), the prostate morphology is less likely to affect candidacy; the Rezūm device can be used to focally treat anatomical variations in the enlarged prostate. Size and morphology should not affect device-related costs as one Rezūm device can be used on prostates of various sizes. In the Rezūm RCT prostates were 30-80 grams1 but clinically, larger prostates have been treated.8 The Rezūm XL trial is currently under way to evaluate efficacy of this procedure in prostates 80-150 grams. However at present, treating prostates larger than
80 grams remains investigational in the United States. Anatomical evaluation using transrectal ultrasound (TRUS), transabdominal ultrasound and cystoscopy can be used for evaluation of surgical candidacy and for surgical planning, as described in American Urological Association (AUA) guidelines.12
Functional studies, patient selection, and patient counseling
Urodynamic studies are helpful in assessing appropriate patients for treatment, but this practice is has not been uniformly recommended prior to proceeding with surgical therapy based on current AUA guidelines for management of BPH.12 However, if more information is needed to determine if surgery or a MIST procedure is best, then functional urodynamic studies are very helpful. Patients in urinary retention often have poor bladder contractility (inadequate detrusor contractility/under active bladder). Retention patients may still be considered for treatment with the Rezūm procedure if functional studies demonstrate adequate functional bladder contractility. Pressure-flow studies measured with formal urodynamics or even a limited noninvasive cystometrogram with Urocuff TEST (SRS Medical, N. Billerica, MA, USA) help stratify patient candidacy and counseling. High pressure voiding (obstruction, with no retention) and anatomical obstruction by the enlarged prostate can be considered for Rezūm or surgery, depending on the patient’s goals and treatment expectations. For example, in a sexually active man with obstruction by an intravesical median lobe, he may opt for treatment with Rezūm to improve LUTS and decrease the likelihood of ejaculatory dysfunction. The incidence rates of bladder neck contracture with surgical procedures of TURP and PVP are reported as being similar, relatively low but may require reoperation.13 It is not clear if preserving the prostatic urothelium with a Rezūm procedure decreases the risk of bladder neck contracture. Comparative studies are warranted.
While excluded from the Rezūm RCT, clinical practice and post-market studies have included patients on anticoagulants and antiplatelet therapy.8 Risk stratification of men on blood thinners is needed in the shared decision making with the patient and cardiologist. If stopping the anticoagulant or antiplatelet therapy is felt to significantly increase cardiovascular/cerebrovascular events, then the patient should remain on their anticoagulant therapy. Local anesthesia poses less risk than general or spinal anesthesia in an anticoagulated patient, and Rezūm patients can be treated under local anesthesia in most cases. Rezūm procedures are faster than traditional surgical interventions and post-procedural bleeding can typically be controlled with a catheter; there have been no comparative trials in this setting. The nature of Rezūm treatment favors less risk as opposed to traditional TURP surgery in high risk men.
Procedural tips – local anesthesia
The Rezūm thermal therapy procedure is well suited to be performed in an office or ambulatory outpatient treatment setting with management of discomfort/pain and anxiety based on shared decision making between the patient and urologist. Each patient’s experience is unique. In prior trials, oral sedation only was used predominantly for anesthesia (69% of patients) as well as prostate block (21%) and intravenous sedation (10%) followed by post-treatment analgesics.1,4
During our early commercial experience, we observed urologists using a modified peri-prostatic nerve block during Rezūm procedures that consistently provided patients with a comfortable treatment experience; modifications are attributed to J.R. Beahrs, MD. A traditional periprostatic block includes bilateral injections of a local anesthetic at the vascular pedicle, the junction between the base of the prostate and the seminal vesicles (sometimes referred to as the “White Mountain” as viewed on TRUS), and along the prostate to the apex. Table 1 presents pain management options used successfully for the Rezūm procedure; Figure 3 illustrates the periprostatic block. No single anesthesia protocol is considered standard. Some patients prefer to not have transurethral or transrectal prostate blocks while completely awake. For patients who prefer sedation, we often work with anesthesiologists to offer in-office or ambulatory surgery center sedation/total intravenous anesthesia with or without the modified prostate block.
Procedure overview
For the water vapor thermal therapy procedure, the creation of continuous, overlapping ablative lesions running parallel to the natural slope of the prostatic urethra is critical to the success. How that is accomplished may be affected by the anatomy of the prostate. As described in the Rezūm RCT, treatment begins with the needle tip visually positioned and inserted beginning approximately 1 cm distal to the bladder neck.1 The treatment needle is retracted after each vapor injection and repositioned in 1 cm increments distally from the previous site to the end of the prostatic tissue just proximal to the verumontanum. With each vapor injection, the bulk of the targeted tissue should be treated. This orientation could be altered as vapor treatments are delivered returning to midline and moving back laterally to identify proper location. For a majority of cases, this pattern of treatment produces a smooth case with optimal outcome. One should complete all treatments on one side of the gland to take advantage of the latent heat from prior treatments on that side. When satisfied, proceed to treat the contralateral tissue of the gland. The total number of vapor treatments in each lobe of the prostate is determined by the length of the hypertrophied prostatic tissue and can be customized to the configuration of the gland including the median lobe as well as protruding intravesical prostate tissue.
Procedural tips: basic surgical techniques
Therapy is performed with the patient in the dorsal lithotomy position; the treatment delivery device (with retractable vapor needle) is inserted into the urethra. As with all procedures, careful attention to position allows for delivery device insertion. Confirmation of the contours of the prostate and planned disbursement of thermal lesions as derived from baseline cystoscopy is the appropriate first step. Paying close attention to the urethral angle obtained on TRUS as well as the intraprostatic structure seen with flexible cystoscopy helps identify challenging anatomic variants such as a protruding median lobe prior to initiating treatment.
Examination of the bladder and the ureteral orifices is important, particularly in the event that median-lobe tissue has elevated the orifice. Specifically, an elevated urethral angle suggests enlargement of the central tissue. Rigid cystoscopy may be difficult to maneuver over the bladder neck, particularly with poor positioning of the patient at the edge of the treatment table. Ensuring a good lithotomy position with the buttocks just off the edge of the table as well as a clear area beneath the table of any obstruction allows for optimal device movement and delivery of the vapor therapy. During the procedure continuous saline irrigation (at room temperature) enhances visualization and cools the urethral surface to preserve the urethral lining. Occasionally the device will not advance into the bladder. No instrument should ever be forced or tearing of the prostate tissue and unwanted bleeding ensues. In this situation, start treatment at the level of the veru on either side of the prostate apex and this will relax the central elevation to allow passage of the device into the bladder, and then continue treatment as described 1 cm from the bladder neck.
Other anatomic variations necessitate alterations of treatment plans. With elevated central tissue or taller prostates, either a Z-type pattern or “stacked lines” (i.e. two separate lines of therapy) are recommended to adequately treat the patient. Encountering median lobe tissue or intravesical growth of the median lobe or lateral lobes requires alternative methods to treat these anatomic structures. For median lobe tissue, entering from a point midway from the base to apex at a 45 degree angle is most useful. For most median lobes, two treatments are adequate. With larger protrusions, a stacked treatment may be required starting about 0.5 cm
from the distal aspect of the protrusion and working back to the base in 1 cm increments allows for complete treatment. Wider based median lobes may also require a single treatment placed at the apex of the median lobe to ensure complete treatment of the median lobe tissue. This same principle should also be applied to lateral lobe prostatic intravesical protrusion. Rather than starting at the bladder neck, the treatment is measured from the tip of the intraprostatic protrusion continuing as discussed above with either a single line, Z-type treatment, or stacked treatment as prostatic anatomy dictates. When treating intravesical segments of the prostate, it is recommended to counsel patients that during recovery they may see tissue fragments pass in the urine as the tissue necrosis process occurs. There is a possibility that retained tissue could lead to bladder stone formation, although this was not observed in the Rezūm RCT. We have confirmed residual tissue with calcification in 3 or 4 patients (< 1%), some of which were discovered 1 year after the procedure. Subsequently if a patient reports passing sloughed tissue, it is recommended to perform a cystoscopy at 3 months to rule out residual tissue. In rare cases of patients with residual urgency and frequency, adherent necrotic tissue could remain within the prostatic fossa and require removal resolving the symptoms.
With convective heat transfer and phase change for delivery of the therapeutic energy, ensuring complete delivery and absorption of the energy is important. After placement of the vapor needle, encouraging the patient to remain still if they are under local anesthesia, and as well, minimizing surgeon movement is important to prevent vapor leak. With vapor leak, incomplete treatment of the tissues may lead to irregular defects and reduced efficacy. If there is a vapor leak characterized by bubbles passing alongside the needle puncture during a treatment, a slight positional adjustment of the treatment needle toward the gap can often stop the leak. Additionally waiting 1 to 2 seconds after completion of the 9-second vapor injection allows for complete phase change and no loss of vapor through the treatment puncture.
Procedural tips: treating patients in urinary retention
Treating patients in urinary retention managed either by clean intermittent catheterization (CIC) or with an indwelling Foley catheter is feasible and successful with careful patient selection. In this situation, it is recommended to perform urodynamic studies prior to proceeding to ensure detrusor function is not impaired enough to prevent emptying once the prostate obstruction is relieved. Flexible cystoscopy and prostate ultrasound represent further baseline evaluations. Obviously, these patients need complete sterilization of bacteriuria prior to Rezūm treatment. Prolonged catheter management — either continuous or CIC— following treatment is critical to allow post-procedural bladder drainage. Persistent catheter drainage for approximately 1 month following the procedure is typical in those who present in urinary retention. This is based on timing of symptom improvement observed as early as within 1 month in the Rezūm pivotal trial. During the acute inflammatory period following treatment, it would not be expected to see resolution of obstruction until a month or more. If the patient fails trial of void at 1 month, consider repeat voiding trials at 1 to 2 week intervals or management with CIC and tracking residual volumes.
Rezūm for patients previously treated with another procedure
In clinical practice some patients have undergone prior procedures such as TURP, PUL, PVP or other BPH treatment. Given the prostate continues to grow even after local therapy, this tissue should respond well to a Rezūm procedure if anatomic and functional studies support that focal debulking with steam may alleviate obstruction. For example, if there is apical nodular re-growth years following TURP seen on cystoscopy and causing functional obstruction, this has been successfully treated with focal Rezūm procedure in those areas. With the flexibility of the Rezūm system to customize the treatment to the patient, steam can be used to focally treat regrowth of tissue anywhere within the prostatic fossa. This can also be applied with prior PUL. However, when a patient has had a prior PUL, there may be an increased risk of exposure of the internal implant stainless steel tab. It is recommended to perform a post treatment cystoscopy at 3 months to ensure no foreign body is present within the prostate fossa that would need to be removed due to the risk of encrustation. Patients with prior radiation have been intentionally avoided due to the loss of the intracellular spaces replaced by scar tissue.
The retreatment rate for Rezūm RCT patients over 3 years of follow up was reported to be 4.4% (6 of 135 patients) including 2 patients with Rezūm, 3 with TURP, 1 prostatectomy. Four of these six secondary interventions were related to the presence of a median lobe, identified but not previously treated.5 Given that no permanent implant is placed, as with PUL, failure is typically due to residual tissue and there is no hesitation to consider repeat Rezūm treatment of patients with suboptimal results after appropriate evaluation. At a minimum, repeat cystoscopy to confirm the presence and location of residual obstructive tissue or potentially inadequate treatment due to vapor leak. If there is any concern for bladder dysfunction, we recommend urodynamic studies prior to proceeding with repeat treatment to ensure no myogenic dysfunction.
Post-procedure care and follow up
In our clinical practice, postoperative management has evolved as experience was gained with the procedure. The postoperative course is usually characterized by 2 to 3 weeks of urgency, frequency, and dysuria. This is driven by the acute inflammatory response to the damaged (ablated) tissue. Typical follow up after the procedure includes a voiding trial at 3 to 7 days, depending on the length of time the catheter is placed. Duration of catheter placement is determined by the volume of tissue ablated and the patient’s ability to empty prior to treatment. For patients who receive less than four vapor injections, a voiding trial can be considered between 1 to 3 days after the procedure. However, for those patients who receive more than six water vapor injections or have a PVR greater than 150 cc are at high risk for postoperative retention. Placement of a Foley catheter for 5 to 7 days may be considered but this does increase the risk of post procedural infection due to colonization of the urinary tract; prophylactic antibiotics can be considered at the time of catheter removal. Return visits at 1 month and 3 months are recommended to ensure continued improvement in symptoms. Most patients are on alpha blockers at the time of treatment and are instructed to wean the medication 3 to 4 weeks after the procedure when symptoms have improved more than baseline.
Initially, we give ibuprofen 800 mg TID for a 2 week course if no contraindications existed, as well as a urinary analgesic/antispasmodic like methenamine 118 mg, sodium phosphate monobasic 40.8 mg, phenyl salicylate 36 mg, methylene blue 10 mg, hyoscyamine sulfate 0.12 mg (Uribel or Urogesic Blue) as needed for dysuria. We have also utilized meloxicam (Mobic) 15 mg daily for ease of dosing but may not have the same symptomatic benefit ibuprofen provided. More recently, we have decreased the ibuprofen dose to 600 mg TID for a 10 day course to mirror the inflammatory cascade with continued success with lower dosing.
With initial treatments a Foley catheter was left for bladder drainage for 3 days and this provided a low postoperative retention rate of 3.7% reported in the Rezūm pivotal trial.1 For some time we also managed patients with no catheter and instructed them on CIC post procedure. If a patient was able to place a catheter, we sent them home with a supply of catheters in case they were unable to empty their bladder. This leads to increased irritative symptoms and complications with UTI and over time we have moved back to an indwelling catheter for 3 days. Since this change, the complications and patient discomfort has significantly reduced. The inflammation experienced immediately after treatment with Rezūm can make self-catheterization difficult. When treating patients with larger residuals or some impaired bladder function leaving a catheter for a longer period of time, 5 to 7 days, would be helpful to reduce the chance of postoperative retention.
Persistent irritative symptoms may occur and when symptoms have not improved after 2 weeks, we check for infection. In these situations, we recommend obtaining a urine culture and treat positive cultures in these patients. When cultures are negative, a Medrol dose pack can be used to reduce inflammation and can be helpful to ease the post procedural irritative symptoms.
Discussion
The convective water vapor energy technology for the Rezūm procedure is a simple, yet powerful new platform technology. It utilizes stored thermal energy of water vapor (steam) created with RF power to result in instantaneous cell death in targeted prostate tissue to alleviate bothersome LUTS resulting from prostatic enlargement. The Rezūm procedure provides versatility for application to a variety of prostate gland morphologies including the median lobe or enlarged central zone. We have been able to offer this minimally invasive treatment option to men disenchanted with suboptimal LUTS improvements on medical therapy or facing the need for long term medical therapy, and in selected patients as an alternative to more invasive surgical approaches. The convenience of performing the procedure without general anesthesia and in an ambulatory outpatient setting has enhanced our practice efficiency. We have successfully treated patients with minimal, transient perioperative side effects. Patients have reported satisfaction with significant early urinary symptom relief and preservation of their sexual functions that reflects the treatment outcomes in the published clinical studies supporting the development of this technology. Setting patient expectations and periprocedural pathways should help patient and surgeon satisfaction with this novel technique.
Conclusions
The Rezūm system has been widely adopted into urology practices in the USA and Europe. It provides the urologist with an efficient, rapid treatment as an office or ambulatory outpatient procedure with minimal transient perioperative side effects, effective and durable alleviation of urinary symptom and a favorable safety profile including preservation of erectile function. This office-based, rapid-delivery MIST with minimal learning curve is amenable to targeted ablation of all zones of the prostate. The water vapor thermal therapy warrants positioning as a procedure for LUTS relief, both as an initial therapy versus medications and as an alternative to transurethral surgery for selected patients.
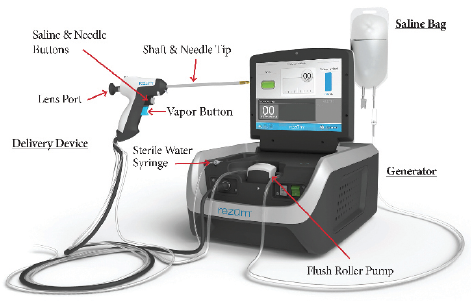
Figure 1.The Rezūm system consists of a Generator with RF power supply that is reusable and is non-sterile (does not come in contact with the patient) and the Delivery Device, a single use disposable device that is provided sterile. Courtesy of Boston Scientific Company, Inc.
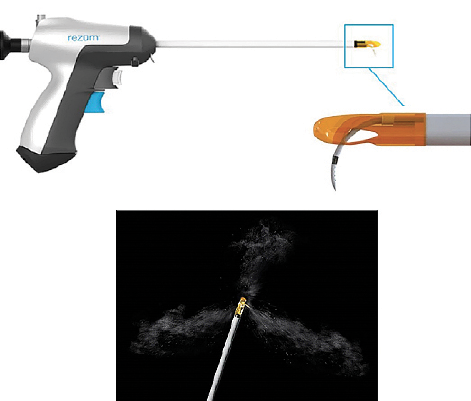
Figure 2. The Rezūm Delivery Device and Vapor Needle. The vapor needle resides within the insulated lumen of the delivery device until it is deployed into the prostate tissue. The hand-held control delivers water vapor providing a consistent energy dose into the prostate tissue through the retractable 18-gauge rigid plastic needle; saline flush irrigation enhances visualization and cools the urethral surface. The needle is flexible braided silicone tubing with 12 small emitter holes spaced around its tip at 120° intervals to allow a controlled, uniform circumferential dispersion of water vapor into the prostate tissue to create an approximate 1.5 cm-2 cm lesion. The insert shows the shaft with the needle deployed. Multiple thermal treatments are delivered with the retractable vapor needle. The length of needle that exits the shaft and penetrates the prostate is 10.25 mm in length. Courtesy of Boston Scientific Company, Inc.
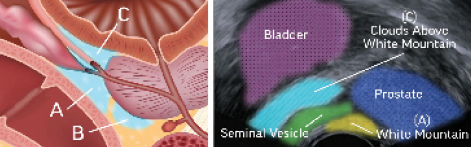
Figure 3. These illustrations of the periprostatic nerve block technique (as outlined in Table 1) are for informational purposes and intended for use only by those physicians who express interest in using this technique. Area between the prostate and the seminal vesicles, or “White Mountain” (A), between the prostate and the rectum (B), area between the prostate and the bladder neck, or the “Clouds Above the White Mountain” (C). Physicians should use their clinical judgment and experience when deciding how to treat patients as each patient experience is unique. Courtesy of Boston Scientific Company, Inc.
TABLE 1. Pain management options for Rezum procedures
|
ORAL MEDICATIONS ONE HOUR PRIOR TO PROCEDURE
PROSTATE BLOCK (see illustrations in Figure 3) SUPPLIES NEEDED
TRADITIONAL BLOCK
MODIFIED PROSTATE BLOCK (Dr. J.R. Beahrs) ADDITIONAL MODIFICATION
To minimize risks associated with infection or bleeding:
Pain management – belladonna and opium 16.2 mg/30 mg suppository at the time of prostate block IV SEDATION IV sedation as per institution anesthesia protocol – e.g. IV propofol with monitored office anesthesia |
References
1. McVary KT, Gange SN, Gittelman MC et al. Minimally invasive prostate convective water vapor energy (WAVE) ablation:
A multicenter, randomized, controlled study for treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol 2016;195(5):1529-1538.
2. McVary KT, Gange SN, Gittelman MC et al. Erectile and ejaculatory function preserved with convective water vapor energy treatment of LUTS secondary to BPH: randomized controlled study. J Sex Med 2016;139(6):924-933.
3. Dixon CM, Cedano ER, Pacik D et al. Two-year results after convective water vapor energy treatment of symptomatic benign prostatic hyperplasia. Res Rep Urol 2016;8:207-216.
4. Roehrborn CG, Gange SN, Gittelman MC et al. Convective water vapor energy (WAVE) ablation therapy: durable two-year results and prospective blinded crossover study for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. J Urol 2017;197(6):1507-1516.
5. McVary KT, Roehrborn CG. Three-year outcomes of the prospective, randomized controlled Rezūm System study: convective radiofrequency thermal therapy for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Urology 2018:111:1-9.
6. McVary KT, Rogers T, Roehrborn CG. Rezūm water vapor thermal therapy for lower urinary tract symptoms associated with benign prosttatic hyperplasia: 4-year results from randomized controlled study. Urology 2019;126:171-179.
7. Woo HH, Gonzalez RR. Perspective on the Rezūm system: a minimally invasive treatment strategy for benign prostatic hyperplasia using convective radiofrequency water vapor thermal therapy. Med Dev (Auckl) 2017;10:71-80.
8. Darson MF, Alexander EE, Schiffman ZJ et al. Procedural techniques and multicenter post-market experience using minimally invasive convective radiofrequency thermal therapy with Rezūm system for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Res Rep Urol 2017;9:159-168.
9. Dixon CM, Cedano ER, Mynderse LA et al. Transurethral convection water vapor as a treatment for lower urinary tract symptomology due to benign prostatic hyperplasia using the Rezūm® system: evaluation of acute ablative capabilities in the human prostate. Res Rep Urol 2015;7:13-18.
10. Mynderse LA, Hanson D, Robb RA et al. Rezūm system water vapor treatment for lower urinary tract symptoms/benign prostatic hyperplasia: Validation of convective thermal energy transfer and characterization with magnetic resonance imaging and 3-dimensional renderings. Urology 2015;86(1):122-127.
11. McNeal JE. The zonal anatomy of the prostate. Prostate 1981;2:
35-49.
12. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline. http://www.auanet.org/guidelines/benign-prostatic-hyperplasia/lower-urinary-tract-symptoms-(2018). Accessed September 19, 2018.
13. Zhou Y, Xue B, Mohammad NA et al. Greenlight high-performance system (HPS) 120-W laser vaporization versus transurethral resection of the prostate for the treatment of benign prostatic hyperplasia: a meta-analysis of the published results of randomized controlled trials. Lasers Med Sci 2016;3:485-495.

