Gilling et al was first to describe the holmium laser resection of the prostate (HoLRP) technique in using the holmium laser for benign prostatic hyperplasia (BPH).1 The HoLRP technique has now evolved into the holmium laser enucleation of the prostate (HoLEP) with the advent of the intravesical soft-tissue morcellator. The HoLEP technique involves anatomically dissecting the prostatic lobes off the surgical capsule in a retrograde fashion. Once the lobes are enucleated, the prostatic tissue is removed from the bladder with an intravesical soft-tissue morcellator. Multiple randomized control trials have shown that HoLEP is associated with a shorter length of catheter use, lower transfusion rates, and a shorter hospital stay when compared to traditional monopolar transurethral resection of the prostate (TURP).2-5 A recent meta-analysis comparing TURP with other minimally invasive surgical therapies found that only HoLEP had a statistically greater mean decrease in International Prostate Symptom Score (IPSS) and a statistically greater increase in maximum urinary flow rate (Qmax).6 HoLEP has even been shown in two randomized trials to have similar results to open prostatectomy with regards to maximum flow rate and IPSS score. In these studies patients undergoing HoLEP had a much shorter length of catheterization, lower transfusion rates, and shorter hospital stay.7,8 Overall, the durability of the HoLEP procedure appears to compare favorably to TURP with results reported including up to 6 years of follow up.9
Equipment
At our institution, we use a 26 French (Fr) continuous flow resectoscope with a laser bridge adapter and an endoscopic camera. The laser bridge adapter stabilizes the laser fiber and fixes it at the 6’oclock position on the endoscope. The laser fiber is passed through a 6 Fr open-ended ureteral catheter with a silicone membrane adapter at the end of the catheter, Figure 1a. This set up helps keep the laser fiber at a fixed length from the tip of the endoscope and stabilizes the laser fiber. We use a 100 Watt holmium laser and an end-firing 550 micron laser fiber with energy settings of 2.0 J and frequency settings of 50 Hz. An 80 Watt or 60 Watt holmium laser can be used, however the lower power setting will increase operative time.
For tissue morcellation, a 26 Fr offset nephroscope is used along with a transurethral soft-tissue morcellator, Figure 1b and 1c. The morcellator consists of two 5 mm reciprocating hollow metal blades, a hand-piece, a two-phase foot pedal, and a control box. The control box consists of a motor unit that powers the blades and supplies suction for tissue removal. A tissue collection device collects the chips as they are suctioned out of the bladder. Small chips retained in the bladder after morcellation may be removed with a modified resectoscope loop, a rigid grasper for the nephroscope, an Ellik evacuator or a glass Toomey syringe.
Enucleation of the prostate
First the 26 Fr continuous flow endoscope with the laser bridge adapter is placed into the bladder. The laser fiber is then passed through the silicone membrane adapter and ureteral catheter which is placed inside the channel of the laser bridge adapter. The fiber tip is kept at a fixed length and fixed location at the tip of the endoscope. This set up allows the surgeon’s hands to be free from holding the laser fiber. The surgeon holds the external endoscopic camera with one hand while the other hand is free to rotate, move and torque the endoscope during enucleation. The outflow port is placed to gravity and the inflow port is wide open infusing normal saline solution.
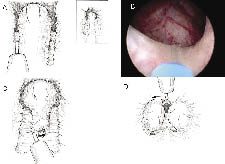
Incisions are made at the 5 o’clock and 7 o’clock positions starting at the bladder neck in the crease between the median and lateral lobes, Figure 2a. The incisions are carried down through the adenoma until the circular fibers of the prostatic capsule are visualized. These incisions are carried distally to the level of the verumontanum. The scope should be torqued downward into the adenoma to effectively split the tissue. These incisions are widened on the surgical capsule, thus isolating the median lobe prior to retrograde enucleation of this lobe. Hemostasis is further accomplished by moving the laser fiber tip away from the tissue and watching the tissue blanch. By holding the fiber away from the tissue, vaporization is avoided and coagulation is achieved. Next the 5 o’clock and 7 o’clock incisions are joined just proximal to the verumontanum. Starting distally, the median lobe is then dissected off the capsule, Figure 2c. The beak of the endoscope is used to mechanically push the tissue off the capsule, helping to define the plane between the median lobe and the prostatic capsule. The holmium laser is used to develop this plane as the median lobe is separated from the capsule in a distal to proximal direction proceeding toward the bladder neck. Care is taken not to undermine the bladder neck. The beak of the endoscope is then used to push the median lobe up into the bladder and the final prostatic attachments are released from the bladder neck allowing the median lobe to fall free floating into the bladder.
The lateral lobes are enucleated one at a time, Figure 2d. The left lateral lobe dissection starts with an incision at the 1 o’clock position. This incision starts at the bladder neck and ends at the level of the verumontanum. This incision is carried through the adenoma until the fibers of the prostatic capsule are visualized. The incision is widened at the level of the surgical capsule by focusing the laser energy at the medial junction between the capsule and the lateral lobe. This is accomplished by rotating the scope in a clockwise fashion. As the incision is widened, the left lateral lobe will start to fall medially into the prostatic fossa. Next, the 5 o’clock incision is extended laterally along the capsule at the apex of the prostate. Then, the 1 o’clock incision is connected to the 5 o’clock distally at the level of the verumontanum. The left lateral lobe is then enucleated off the surgical capsule by using the beak of the endoscope to push away the adenoma off the capsule and exposing the correct plane. The lateral lobe is released at the level of the bladder neck allowing it to fall free floating into the bladder.
The right lateral lobe dissection is accomplished in a similar manner. The dissection starts with an incision at the 11 o’clock position. Again, this incision starts at the bladder neck and ends at the level of the verumontanum. It is carried through the adenoma until the fibers of the prostatic capsule are visualized. As above, this incision is widened at the level of the surgical capsule by focusing the laser energy at the medial junction between the capsule and the lateral lobe. This is accomplished by rotating the scope in a counterclockwise fashion while widening the incision. The lateral lobe will start to fall medially into the prostatic fossa. Then, the 7 o’clock incision is extended laterally along the capsule at the apex of the prostate. Next, the 11 o’clock incision is connected to the 7 o’clock distally at the level of the verumontanum. The lobe is then enucleated off the surgical capsule by using the beak of the endoscope to push the adenoma off the capsule and expose the correct plane. The lateral lobe is released at the level of the bladder neck allowing it to fall free floating into the bladder.
Hemostasis is very good with this technique, however persistent small bleeders should be coagulated prior to morcellation. Coagulation is accomplished by “defocusing” the laser energy off any bleeding tissue. The desired result is that the tissue will blanch and stop bleeding.
Tissue morcellation
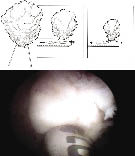
The first step in tissue morcellation is to ensure the bladder is full. A 26 Fr offset nephroscope with the soft-tissue morcellator is inserted into the bladder. The blades of the morcellator should be placed in the center of the bladder away from the bladder mucosa. The two-phase foot pedal is then depressed to engage the suction but not the reciprocating blades. Adenoma is then drawn to the tip of morcellator blades, Figure 3.
If the suction necessary to engage the adenoma begins to collapse the bladder too quickly, a second inflow tubing can be attached to the nephroscope outflow channel to increase the inflow and keep the bladder distended. Once the adenoma is at the tip of the morcellator, the foot pedal is depressed further, activating the blades, and the adenoma is morcellated, cutting the adenoma into small pieces that are suctioned out of the bladder and deposited in the collection chamber. Small pieces of the adenoma should not be chased with the morcellator blades in the bladder because of the increased risk of bladder injury. Instead the small adenomatous tissue should be removed by either a modified resectoscope loop, a rigid grasper for the nephroscope, Ellik evacuator or glass Toomey syringe. Once all chips are removed a 24 Fr three-way Foley catheter is inserted and bladder irrigation is instituted if needed.
If continuous bladder irrigation is used then usually it is continued only overnight. In the am of postoperative day 1 the bladder irrigation is discontinued, and patients are given a trial of void. If they fail the trial of void they are discharged home on postoperative day 1 with an 18 Fr Coude catheter.
Many surgeons are resistant to adopt the HoLEP procedure, stating a steep learning curve, increased operative times and increased costs associated with laser prostatectomy. At our institution, junior residents are exposed to HoLEPs and TURPs with about the same frequency and subjectively the learning curves are similar. Although most published series do report longer operative times for HoLEP versus TURP, differences are on the order of 20-30 minutes longer. However, recent cost analysis have shown that HoLEP is more cost effective that TURP.10
Overall, HoLEP is a well-established alternative to TURP. Outcomes from multiple randomized control trials prove HoLEP is as good as if not better than traditional TURP with regards to length of catheter use, chance of blood transfusions, length of hospital stay, improvement in maximum flow rate, and improvement in IPSS scores.2-5 Complication rates have also been reported to be similar. There are many minimally invasive surgical therapies for benign prostatic enlargement but none of them compare to TURP as favorably as HoLEP.
HoLEP is also a real alternative to open prostatectomy. Multiple authors have reported that for larger prostates, greater than 70 gr to 100 gr, HoLEP provides similar outcomes when compared to open prostatectomy with regards to IPSS score and improvement in maximum flow rate. These authors also reported that length of catheter use and hospital stay were significantly shorter in the HoLEP group, and that complications including blood transfusions were less in the HoLEP group.7,8 At our institution we routinely operate on glands over 100 gr with patients going home on postoperative day 1. As the body of evidence continues to grow in favor of HoLEP, it may become the new gold standard in BPH therapy, replacing both TURP and open prostatectomy.
HoLEP is a real alternative to TURP and open prostatectomy with proven advantages. An anatomic dissection of the prostate from its capsule is attractive to surgeons and patients alike. Once the basics are learned, HoLEP can become a part of any practicing urologist’s armamentarium.

 Indexed in Index Medicus and Medline
Indexed in Index Medicus and Medline